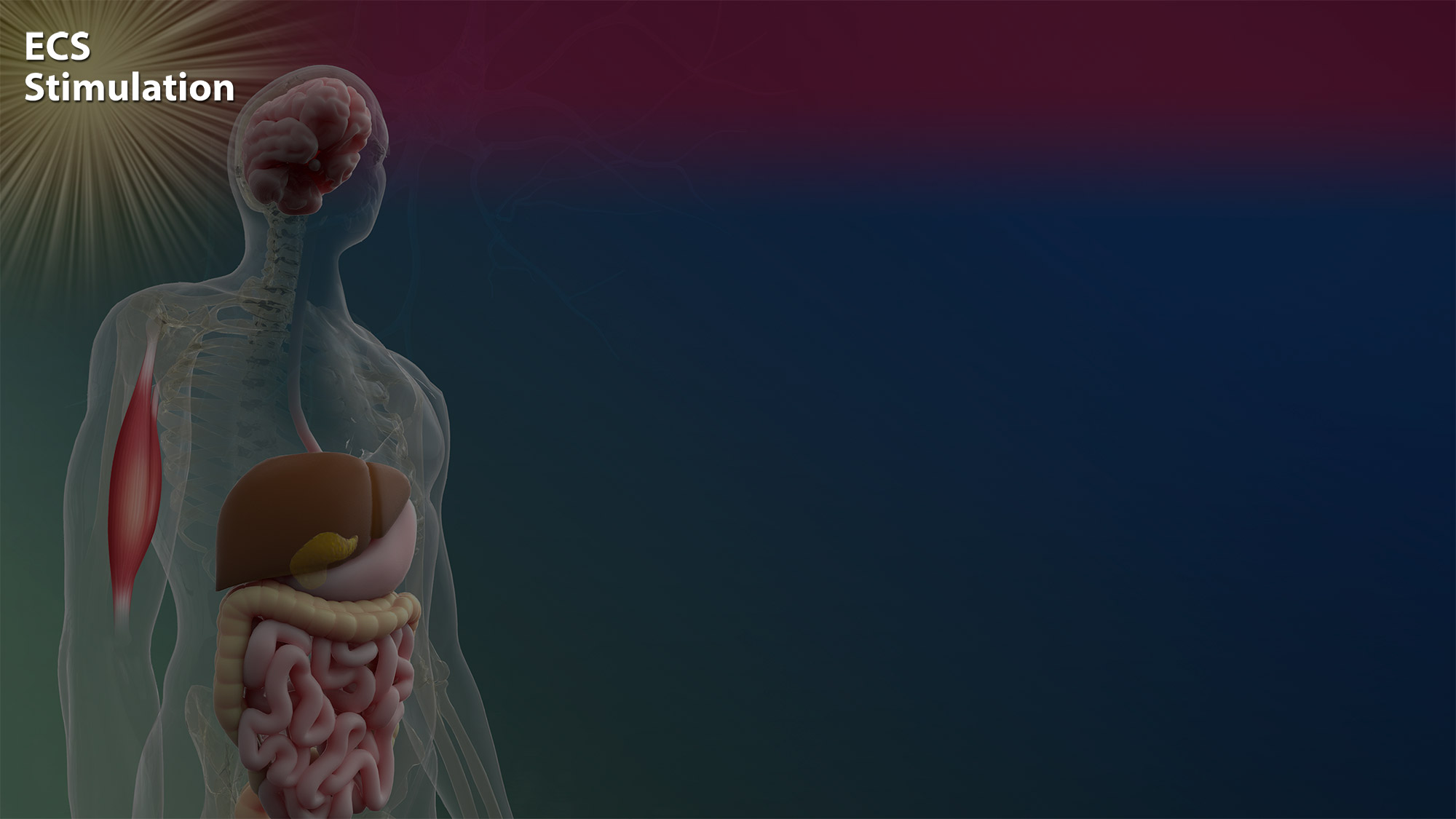



Stimulation of the endocannabinoid system (ECS)
In 1964, researchers isolated the psychoactive ingredient of Cannabis sativa, Δ9-tetrahydrocannabinol (THC). Nearly 30 years later, the endogenous counterparts of THC, collectively termed endocannabinoids, were discovered: N-arachidonoylethanolamine (anandamide, AEA) in 1992 and 2-arachidonoylglycerol (2-AG) in 1995.
Since then, considerable research has shed light on the effect of endocannabinoids on human health and disease, leading to the identification of an ensemble of proteins that bind, synthesize and degrade them –
together forming the ECS. Stimulation of the ECS by different agents controls basic biological processes, including cell choice between survival and death and progenitor or stem cell proliferation and differentiation.
Scroll to begin
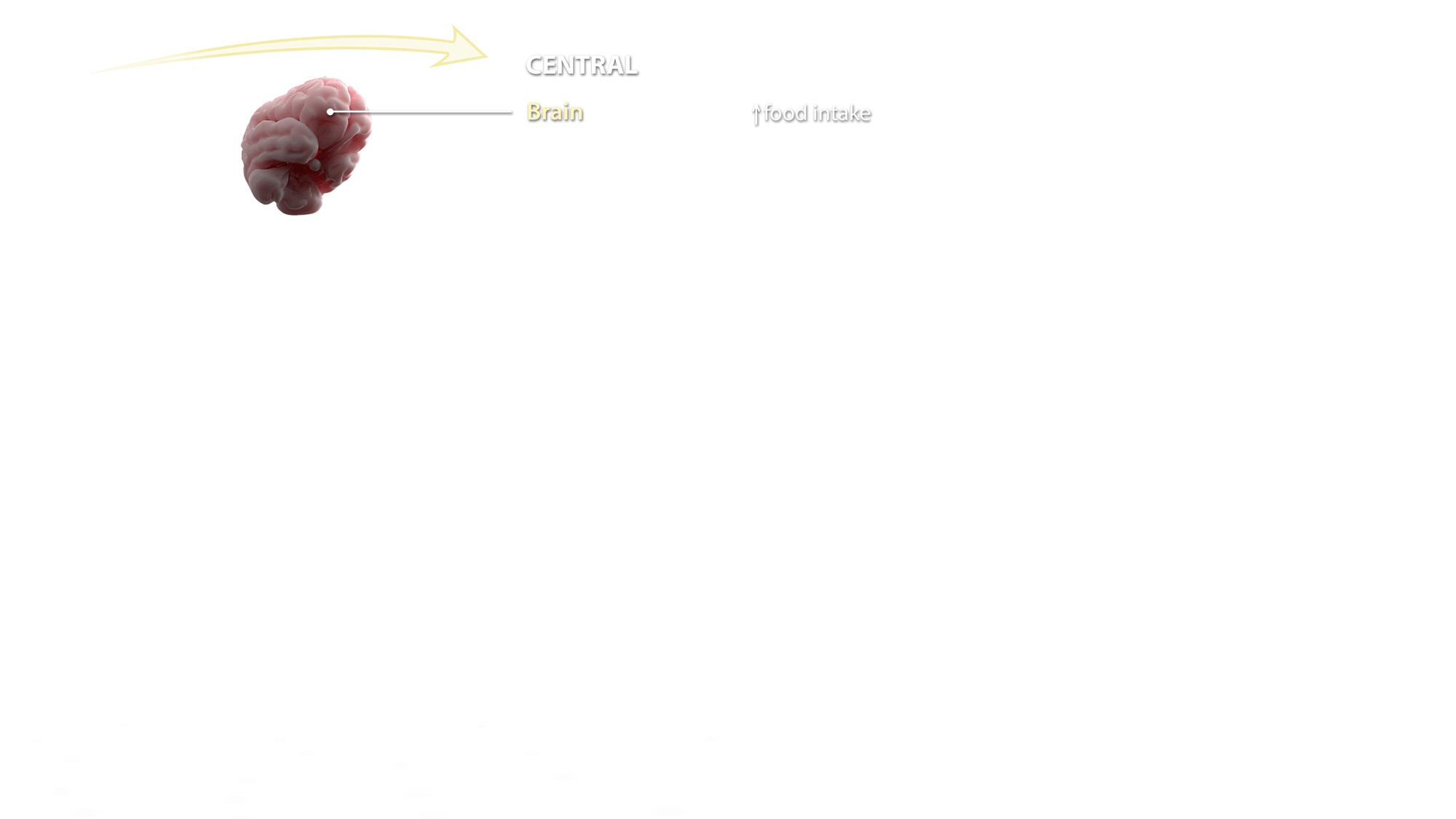
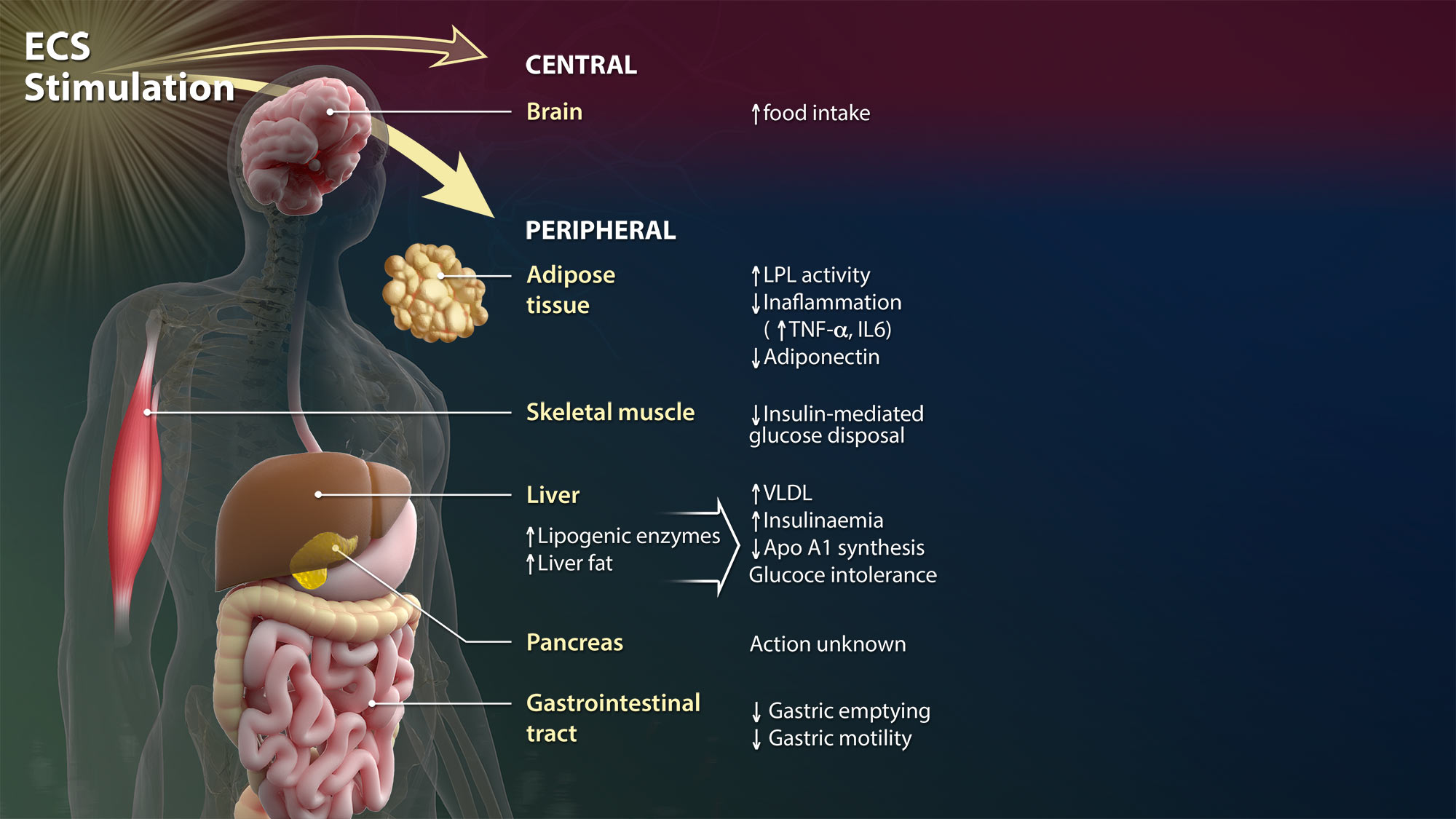
Central
The central nervous system is the complex of nerve tissues that comprises the brain and spinal cord. Here, endocannabinoid signaling is very intense and facilitates cross-talk with virtually all major neurotransmitters (acetylcholine, glutamate, ATP, serotonine, noradrenaline, dopamine, and γ-aminobutyric acid or GABA) to achieve neuromodulation – or it acts directly, leading to an authentic endocannabinoidergic transmission.
Brain
All brain areas have been shown to express a fully functional ECS including at least the following components:
- AEA and 2-AG;
- Their targets type-1 (CB1) and, upon insult, type-2 (CB2) cannabinoid receptors;
- A bunch of metabolic enzymes for the synthesis and degradation of AEA (known as NAPE-PLD and FAAH, respectively) or 2-AG (known as DAGL and MAGL, respectively);
- Putative transmembrane transporters and intracellular carriers.
Altogether, the ECS regulates brain functions that span from control of pain initiation, wake/sleep cycles, thermogenesis and appetite, along with impairment of working memory and memory consolidation, control of psychomotor disorders and of energy homeostasis.
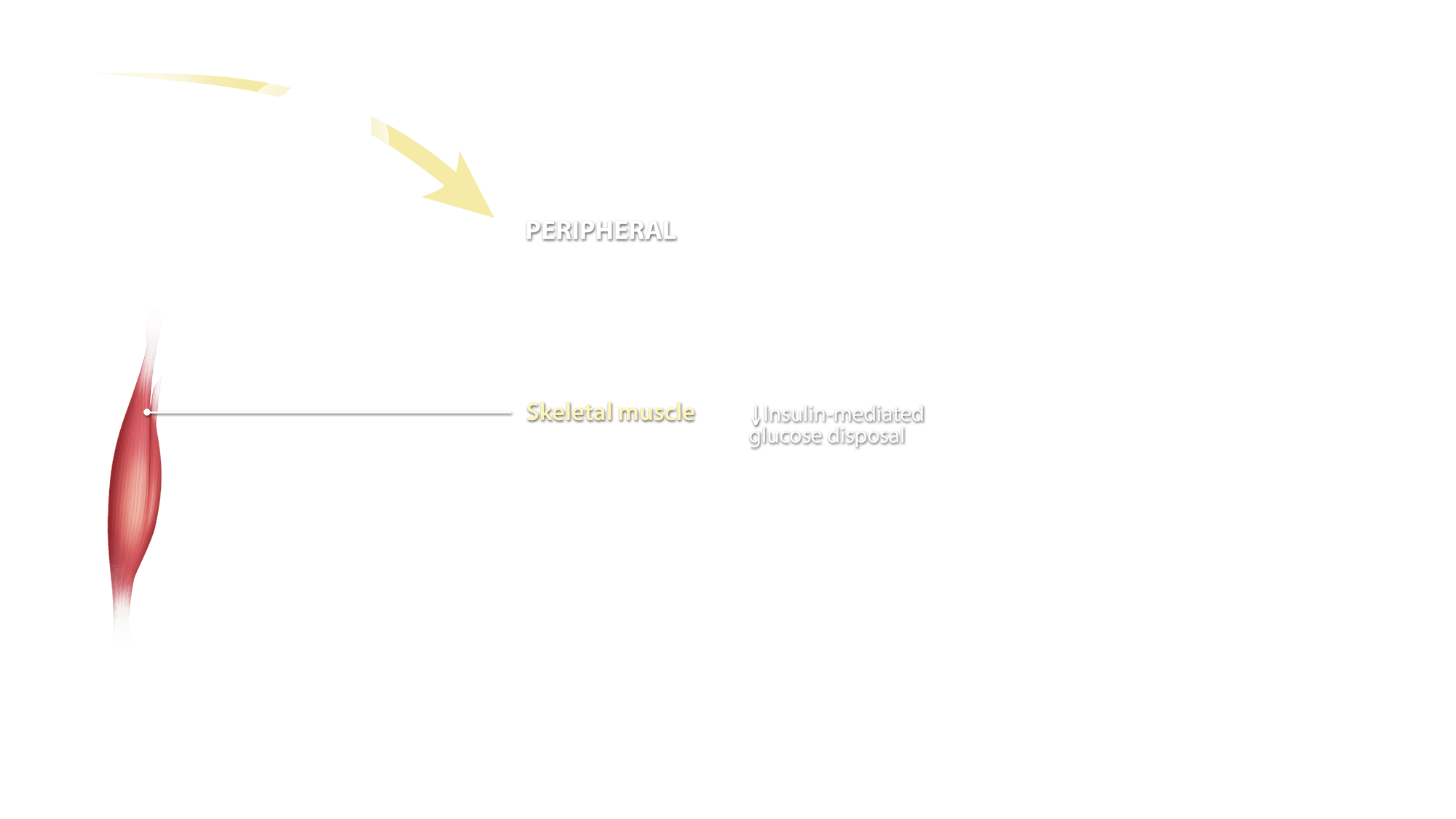
Peripheral
Much like the brain, the ECS is present also at the periphery, where its complexity supports many organ-specific activities and may offer various targets for the development of selective drugs able to modulate endocannabinoid signaling in distinct peripheral cells
Adipose Tissue
ECS activation in adipocytes controls several molecular targets such as adiponectin, interleukins and lipoprotein lipase, overall contributing to the regulation of energy homeostasis, glucose uptake and inflammation.
Skeletal Muscle
Recent data demonstrate that ECS activation controls the skeletal muscle differentiation process, from myoblasts to myotubes, myofibers, and fascicles. It appears that activation of CB1 receptors inhibits myoblast-to-myotube differentiation, whereas blockade of the same receptor subtype facilitates this process and, in vivo, leads to larger fibers.
Liver and pancreas
In the liver, activation of CB1 receptors promotes steatosis, hepatic apoptosis and fibrogenesis, whereas activation of CB2 receptors leads to a reduction in the same processes. Overall, ECS activation in the liver affects insulinaemia, glucose intolerance and energy homeostasis.
Endocannabinoid signaling in the pancreas appears to drive insulin and glucagon release with a clear impact on energy homeostasis and diabetes
Gastrointestinal Tract
Activation of both CB1 and CB2 receptors reduces gut motility, inflammation and immune activation, and regulates food intake through actions on enteroendocrine cells in the gut wall. CB1 receptors also enhance intestinal permeability
Summary
It is apparent that ECS activation regulates many actions both in the central nervous system and at the periphery, thus representing a challenging opportunity to understand how fine-tuning distinct molecular events can drive normal function or pathological states in humans. Yet, complexity and, to some extent, promiscuity of endocannabinoid metabolism and signaling call for attention when aiming at the development of new drugs to combat human diseases.
One should carefully aim to hit the right ECS element in the right place to avoid unwanted side effects that might inhibit the potential benefits of ECS-targeting therapeutics

Artwork by John Karapelou. Text by Phytecs. Art and text are available for use pursuant to a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License. License terms can be found here.
Stimulation of the endocannabinoid system (ECS)
In 1964, researchers isolated the psychoactive ingredient of Cannabis sativa, Δ9-tetrahydrocannabinol (THC). Nearly 30 years later, the endogenous counterparts of THC, collectively termed endocannabinoids, were discovered: N-arachidonoylethanolamine (anandamide, AEA) in 1992 and 2-arachidonoylglycerol (2-AG) in 1995.
Since then, considerable research has shed light on the effect of endocannabinoids on human health and disease, leading to the identification of an ensemble of proteins that bind, synthesize and degrade them –
together forming the ECS. Stimulation of the ECS by different agents controls basic biological processes, including cell choice between survival and death and progenitor or stem cell proliferation and differentiation.
Scroll to begin
Scroll to begin
Central
The central nervous system is the complex of nerve tissues that comprises the brain and spinal cord. Here, endocannabinoid signaling is very intense and facilitates cross-talk with virtually all major neurotransmitters (acetylcholine, glutamate, ATP, serotonine, noradrenaline, dopamine, and γ-aminobutyric acid or GABA) to achieve neuromodulation – or it acts directly, leading to an authentic endocannabinoidergic transmission.
Brain
All brain areas have been shown to express a fully functional ECS including at least the following components:
- AEA and 2-AG;
- Their targets type-1 (CB1) and, upon insult, type-2 (CB2) cannabinoid receptors;
- A bunch of metabolic enzymes for the synthesis and degradation of AEA (known as NAPE-PLD and FAAH, respectively) or 2-AG (known as DAGL and MAGL, respectively);
- Putative transmembrane transporters and intracellular carriers.
Altogether, the ECS regulates brain functions that span from control of pain initiation, wake/sleep cycles, thermogenesis and appetite, along with impairment of working memory and memory consolidation, control of psychomotor disorders and of energy homeostasis.
Peripheral
Much like the brain, the ECS is present also at the periphery, where its complexity supports many organ-specific activities and may offer various targets for the development of selective drugs able to modulate endocannabinoid signaling in distinct peripheral cells
Adipose Tissue
ECS activation in adipocytes controls several molecular targets such as adiponectin, interleukins and lipoprotein lipase, overall contributing to the regulation of energy homeostasis, glucose uptake and inflammation.
Skeletal Muscle
Recent data demonstrate that ECS activation controls the skeletal muscle differentiation process, from myoblasts to myotubes, myofibers, and fascicles. It appears that activation of CB1 receptors inhibits myoblast-to-myotube differentiation, whereas blockade of the same receptor subtype facilitates this process and, in vivo, leads to larger fibers.
Liver and pancreas
In the liver, activation of CB1 receptors promotes steatosis, hepatic apoptosis and fibrogenesis, whereas activation of CB2 receptors leads to a reduction in the same processes. Overall, ECS activation in the liver affects insulinaemia, glucose intolerance and energy homeostasis.
Endocannabinoid signaling in the pancreas appears to drive insulin and glucagon release with a clear impact on energy homeostasis and diabetes
Gastrointestinal Tract
Activation of both CB1 and CB2 receptors reduces gut motility, inflammation and immune activation, and regulates food intake through actions on enteroendocrine cells in the gut wall. CB1 receptors also enhance intestinal permeability
Summary
It is apparent that ECS activation regulates many actions both in the central nervous system and at the periphery, thus representing a challenging opportunity to understand how fine-tuning distinct molecular events can drive normal function or pathological states in humans. Yet, complexity and, to some extent, promiscuity of endocannabinoid metabolism and signaling call for attention when aiming at the development of new drugs to combat human diseases.
One should carefully aim to hit the right ECS element in the right place to avoid unwanted side effects that might inhibit the potential benefits of ECS-targeting therapeutics